Protein–DNA conjugates play essential roles in biotechnology, diagnostics, and molecular imaging. However, DNA-based constructs are susceptible to nuclease degradation, which might limit their effectiveness in complex biological environments, such as cell lysates or live-cell assays. One strategy to overcome this limitation is to use phosphorothioate-modified DNA (psDNA), in which (some of) the non-bridging oxygens in the phosphate backbone are replaced with sulfur:
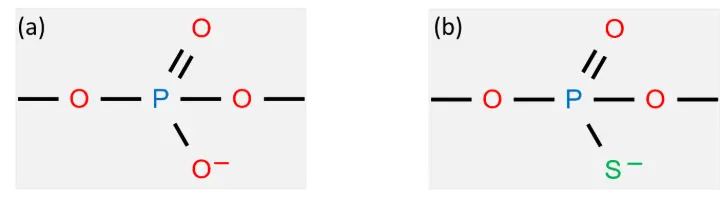
Figure 1. DNA backbone with non-bridging oxygen (a) and a phosphorothioate derivative, psDNA (b)
The presence of sulfur in the backbone raises questions about reactivity and unwanted side reactions, particularly in the context of conjugation of alkyl-thiol-terminated oligonucleotides to maleimide derivatives of proteins. While there are theoretical reasons to suspect lower reactivity of backbone thiols, the extent of any interference was determined experimentally by Rabe & Niemeyer¹ in reactions between either standard oligonucleotides or psDNA also bearing a 5′-terminal alkyl-thiol and maleimide-derivatised streptavidin.
Conjugation was analysed via non-denaturing PAGE and denaturing PAGE, verifying molecular weights, hybridisation ability, and extent of covalent attachment. A nuclease challenge assay was also performed by incubating conjugates with exonuclease I, which digests single-stranded DNA from the 3′ end. Retention of DNA hybridisation and streptavidin-binding ability post-treatment was used as a readout for functional integrity.
Key findings were that both standard and psDNA oligonucleotides (with 18 phosphorothioate linkages) reacted with maleimide-functionalised streptavidin only via the 5′-alkyl-thiol, with no detectable reaction between the maleimide and the backbone sulfur atoms. Thus, psDNA is compatible with the well-established maleimide bioconjugation chemistries.
Moreover, psDNA–streptavidin conjugates successfully hybridised to complementary strands, as assessed in gel shift experiments, and bound biotinylated alkaline phosphatase with high affinity, indicating preserved functional properties. Standard DNA–streptavidin conjugates were rapidly degraded by exonuclease I, losing hybridisation capacity, whereas psDNA conjugates showed near-complete resistance.
In practice, stability to exonuclease is often obtained by adding just 2 or 3 phosphorothioate links at the 3’ end, and the current study, by changing all positions, convincingly demonstrates that backbone non-bridging thiols can be present without affecting standard maleimide reactions. Chemically, this is likely explained by a combination of reduced electron density, resonance stabilisation, and steric hindrance of the backbone thiol compared with terminal alkyl thiols.