Introduction
Imaging Mass Cytometry (IMC) has the potential to profile ~40 proteins in tissues but struggles to detect low-abundance markers. The latest advance—SABER-IMC (Signal Amplification By Exchange Reaction)—solves that sensitivity gap by adding a DNA-engineered amplification layer that sits on top of the antibody binding. The critical reagent tool at the heart of this workflow is the antibody–oligonucleotide conjugate (AOC).
The AOC-first workflow
SABER-IMC converts each antibody binding event into dozens of readable signals through a programmable DNA scaffold:
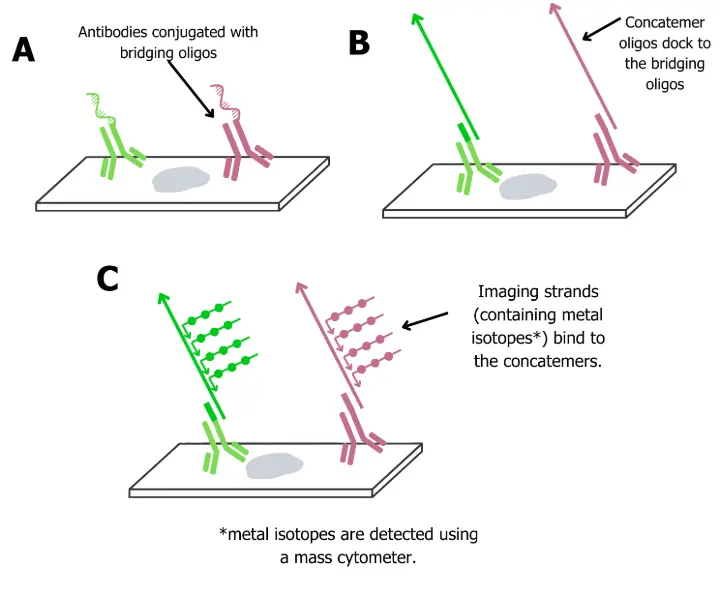
A) The Bridging oligo is conjugated to the antibody
Each antibody carries a unique 42-mer “bridge” oligo. This preserves protein specificity while creating a handle for downstream DNA assembly.
B) Concatemers dock to the bridging oligo
A single-stranded concatemer hybridizes to the bridge at its 5′ end. This concatemer is designed with many repeated 9-mer barcode sites at its 3′ end.
C ) Isotope-tagged imagers
Each 9-mer repeat recruits multiple imager strands, short oligos conjugated to metal isotopes, resulting in signal amplification when read using a mass cytometer.
Tunable amplification (rounds ×1/×2/×3)
Additional concatemer rounds build branched DNA with extra imager binding sites. Therefore scientists have the option to dial sensitivity by choosing either 1, 2 or 3 rounds for each target. In practice, SABER-IMC enables simultaneous imaging of amplified and unamplified panels in a single experiment, capturing rare markers alongside abundant ones without extra imaging cycles.
The benefits of SABER-IMC
Sensitivity for scarce proteins
Low-copy molecules such as PD-1, LAG-3, CTLA4, often undetectable by standard IMC, become visible and quantifiable when their antibodies are equipped with DNA bridges and amplified via SABER.
Scalable multiplexing without host-species limits
Because AOCs use distinct DNA barcodes (not secondary antibodies), many targets can be amplified together with minimal cross-talk from secondary antibodies.
Programmable signal gain
The method reports target-dependent gains on the order of ~8–16× (2 rounds of amplifications) and ~16–68× (3 rounds of amplification) versus standard.
Cleaner, faster runs
SABER-IMC removes the repeated imaging cycles of fluorescence-based amplification while remaining compatible with FFPE tissues and routine IMC workflows.
Technical insights & assay tips
Here are some practical considerations from SABER-IMC that can improve reproducibility and performance when working with AOCs:
- Bridge DNA length matters: The 42-mer design provides enough stability for concatemer docking while minimizing secondary structures. Shorter bridges (<30 nt) risk poor hybridization; longer bridges may fold back and reduce efficiency.
- Concatemer QC: Before using, validate concatemer length by gel electrophoresis. Over-extended concatemers increase background; under-extended concatemers reduce signal gain. Aim for concatemers with consistent size distributions.
- Avoid barcode cross-talk: Distinct 9-mer barcodes should be computationally checked for cross-hybridization. Even single mismatch can lead to off-target imager binding in multiplex panels.
- Optimize SABER rounds by target. Use one round of amplification for abundant structural proteins (e.g., cytokeratins). Two rounds of amplification balances sensitivity and specificity for moderately expressed proteins. Reserve three rounds of amplification for low abundant proteins, for instance, checkpoint-type molecule.
- Metal-isotope imagers: Choose isotopes outside the main antibody-metal panel to avoid spectral overlap. Matching isotope availability with your cytometer’s detection channels prevents wasted targets.
- Blocking steps reduce noise: Pre-hybridization with blocking oligos can prevent sticky concatemer–concatemer interactions and reduce non-specific signals in complex tissues.
- FFPE considerations: Slightly longer hybridization times (up to 3 hours) may be required in FFPE tissues due to crosslinking, compared to frozen sections.
- Batch consistency is critical: Even small variations in oligo:antibody ratio shift amplification outcomes. QC steps such as UV spectrophotometry (for oligo quantitation) and mass photometry (for conjugation homogeneity) should be standard.
The takeaway
Antibody–oligo conjugates transform IMC sensitivity from “can’t see it” to “clear signal,” while preserving the multiplex scale that spatial biology demands. If your questions hinge on scarce markers in complex tissues, AOCs + SABER-IMC are the shortest path to answers.
AbOliGo Custom AOC services - learn more
Reference