Use of Benzoylacrylic-labelled Oligonucleotides to Create Hydrolytically Stable, Site-specific Protein Conjugates
Introduction
Many existing methods for conjugating oligonucleotides to biomolecules suffer from several drawbacks, including a lack of site-specificity (hence formation of heterogeneous mixtures), poor control over stoichiometry, and hydrolytic instability, which can be especially problematic in in vivo applications.
Conjugation Method
Konc et al. (References) describe a new conjugation strategy using a benzoylacrylic acid pentafluorophenyl ester reagent (BA-PFP2). This reagent labels amino-modified oligonucleotides with a benzoylacrylic (BA) tag, which can subsequently react with accessible cysteine residues or other thiols on biomolecules via Michael addition to yield a stable thioether linkage. The study is remarkable for its exceptionally detailed characterization of conjugates by mass spectrometry (see Supplementary Information).
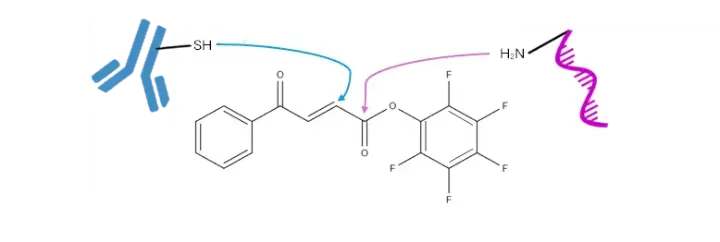
Figure 1. BA-PFP2 linker showing points of attachment of thiol and amine ligands
The PFP ester was chosen over the more commonly used NHS ester due to its superior hydrolytic stability. The BA tag provides an alternative to the maleimide functionality commonly used in bioconjugation reagents. Surprisingly, the BA tag exhibits similar reaction kinetics to maleimide, but with enhanced stability and fewer side reactions.
Single-stranded BA-modified oligonucleotides (11-mer, 25-mer and 50-mer) and a double-stranded 19-mer were reacted with proteins or antibodies bearing a solvent-accessible cysteine residue (native or engineered) under mild conditions (pH ~8, 25–37°C, 30–60 min). The set of proteins included ubiquitin K63C, the C2A domain of synaptotagmin-I C2Am-C95, human serum albumin C34, and annexin V C315, as well as antibodies, including full IgGs (heavy and light chains) with engineered cysteines and nanobodies.
Site-specific Antibody-oligo conjugate Performance
The authors reported advantages of this conjugation technology include greater stability during the oligonucleotide-labeling step and also improved stability of the final conjugate, compared with equivalent maleimide-based reagents. For example, maleimido-propionic acid NHS ester was converted not only to the desired product but also to maleamic acid (the hydrolysis product of maleimide) which prevents any reaction with proteins. Although the conditions used (pH 8) were primarily optimized for BA rather than for maleimide, the data clearly indicate that the BA functionality is more stable. The maleimide-oligonucleotide derivative underwent complete hydrolysis to maleamic acid after 24 h at 37°C, as demonstrated by LC-MS analysis (mass increase of +18 Da, consistent with water addition) rather than cleavage or linker loss. In contrast, the profile for the BA-labeled oligonucleotide remained unchanged under the same incubation conditions.
Stability tests of the DNA-protein/antibody conjugates in human plasma (10% plasma in PBS, pH 7.4, at 37°C) showed that the BA-oligonucleotide-conjugated nanobody (2Rb17c-C138-ss11-mer) remained intact for over 72 h, as confirmed by LC-MS. This result indicates that the BA linkage, and the conjugate itself, is stable in biologically relevant fluids and not subject to retro-Michael addition or thiol-exchange reactions (e.g., with glutathione).
Engineered cysteine residues in therapeutic antibodies were exploited with BA chemistry to achieve site-specific conjugation, as demonstrated with anti-EGFR2 antibody Thiomab LC-V205C and anti-CD33 antibody Gemtuzumab and its variants. Notably, in antibodies lacking additional cysteines, no undesired modification with BA analogues was observed. BA conjugates were also successfully employed in fluorescence and super-resolution microscopy (DNA-PAINT).
Summary
Overall, the benzoylacrylic-based conjugation chemistry overcomes many limitations of traditional maleimide/NHS crosslinkers, particularly regarding hydrolytic stability, a critical factor in some in vivo applications. Unfortunately, unlike with maleimide-based linkers, the BA-PFP2 reagent is not commercially available, though details of the synthesis are given.